9.4. Event Supposedly Attributable to Vaccination or Immunization (ESAVI)
Standard Operating Procedure - Event Supposedly Attributable to Vaccination or Immunization (ESAVI)
Effective Date: [Date]
Version: 1.0
Prepared by: [Name/Department]
Approved by: [Name/Department]
Background
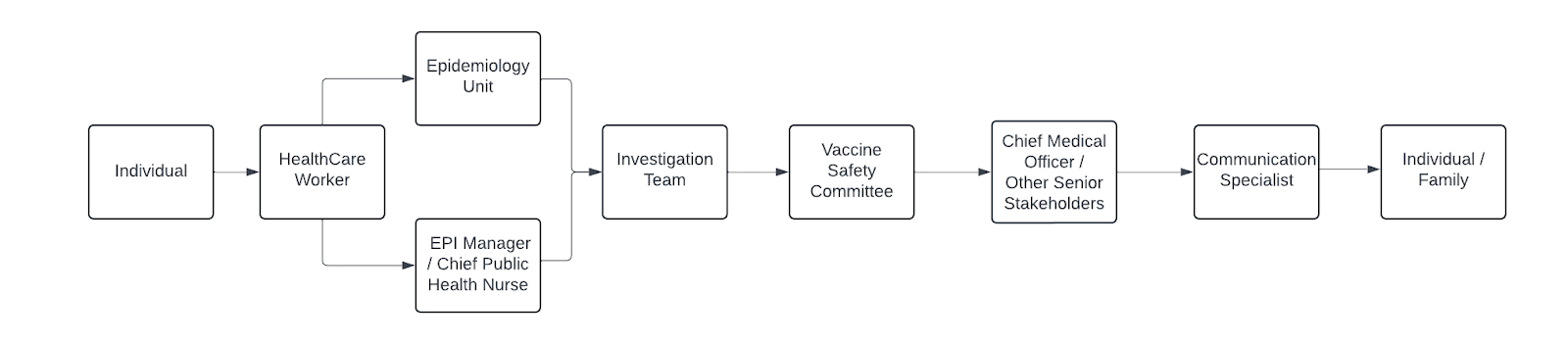
ESAVI surveillance should be done following the ESAVI Surveillance cycle. This cycle consists of six (6) distinct steps including: 1) detection and comprehensive healthcare, 2) notification to the healthcare system, 3) investigation, 4) data analysis, 5) causality assessment and classification and 6) feedback with corrective measures and response. The person experiencing the ESAVI is the center of all activities. Several stakeholders play different roles in ESAVI surveillance. This SOP provides a comprehensive breakdown of each step taken in the realms of each respective role.
Step 1-2. Detection and Notification
Detecting and Reporting ESAVI (Individuals) - Individuals are an essential component to ESAVI Surveillance and should be aware of the country specific procedures / where they can be assisted if they experience an ESAVI. The following is the role of the individual.
- After detecting an ESAVI make a report (the vaccine recipient or the parent of the vaccinated child). (Not all ESAVI require medical attention, some may resolve with no treatment or with only symptomatic treatment.)
- Report all ESAVI (regardless of whether the individual sought medical attention). Individuals should be informed of the platform(s) existing to make this report.
- If an individual-based platform exists for reporting ESAVI, complete the ESAVI form in as much detail as possible including the below mentioned information. If a platform doesn’t exist, the individual or her/his parent/guardian should go to the closest public health centre or the health institution you were vaccinated at to report.:
- Name of the person vaccinated
- Date of birth/Age
- Address
- Contact details
- If applicable; pregnancy and lactation status
- Date and time of vaccination
- Place of vaccination
- Name of the vaccine
- The vaccine batch number
- Dose Number
- The specific adverse events they are experiencing or have experienced
- The date and time the event occurred
- Whether or not the event is still occurring or if they have recovered from it.
- Whether or not they sought, medical attention and if so, which doctor did they see, when did they see the doctor and what was the outcome of that visit.
Note: Contact the individual either by telephone or email for further steps if the event reported is considered to be a Serious ESAVI based on the definition above.
If you are travelling outside of the country where you were vaccinated, then the report should be made to the country the individual was vaccinated.
Detecting ESAVI (Healthcare Worker)
First be focused on the person's health status, to ensure accurate diagnosis and effective treatment, as well as any necessary rehabilitation measures.
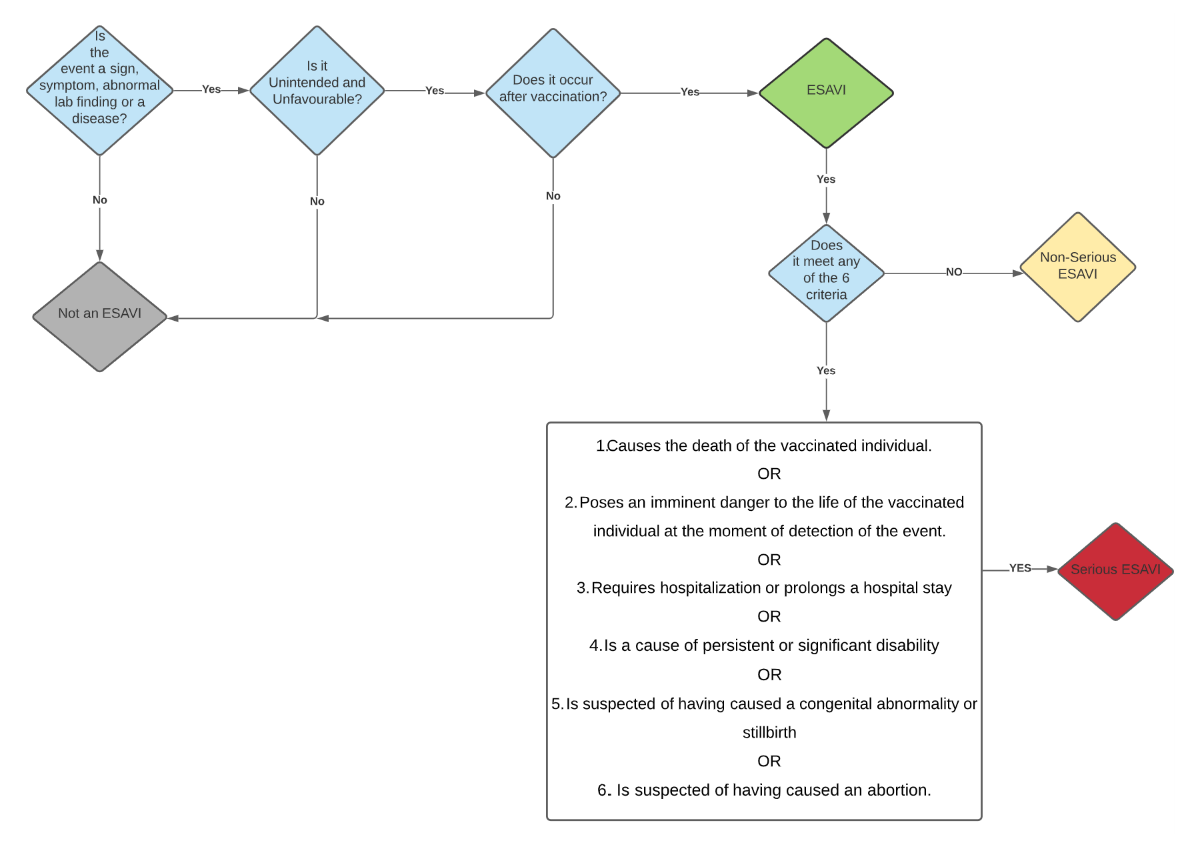
Determine if this event should be considered an ESAVI based on the definition.
Classify the ESAVI into one of two categories: Serious ESAVI or Non-Serious ESAVI. Use the decision tree provided below in Figure 1 to assist in categorizing the ESAVI.
Regardless of the classification, report the ESAVI (details in next step).
Reporting ESAVI (Healthcare Worker)
- Report any ESAVI case to the surveillance unit using ESAVI reporting form.
- All ESAVI should be reported regardless of its classification.
- Note: Any change in the health of a vaccinated person within the first 30 days after administration should be suspected of being the result of administration of the vaccine; however, the occurrence of an event after 30 days does not exclude the possibility of its being associated with the vaccine.
- Report all serious ESAVI within the first 48 hours after detection, ideally immediately.
- Report all non-serious ESAVI within the first 7 days of detection.
- Simultaneously, where possible, share the information reported to surveillance unit with the Chief Public Health Nurse and EPI Manager as well.
- When reporting, use clinical case definitions based on the Brighton Collaboration definitions. These can be found here: https://brightoncollaboration.us/category/pubs-tools/case-definitions/
- Report as much information as possible for proper follow-up and if an investigation is warranted or further information is needed.
Complete the form in as much detail as possible. Ensure that you include:
The ESAVI reporting ID
The date the patient came to the healthcare system
The date of the report
The vaccine name
The vaccine manufacturer
The vaccine batch number
If applicable, any diluent information
The date and time of the vaccination
The dose number
The adverse events
A description of the signs, symptoms, abnormal laboratory findings or disease
The date and time the ESAVI started
The ESAVI classification (Serious vs Non-Serious)
The outcome of event (example recovered or not recovered)
All relevant medical history including pregnancy details as relevant
The Patient details, inclusive of name, date of birth, sex, address and contact details
Name and address of the health facility/ vaccination site
The reporter’s name and contact details.
Data Management (Epidemiology Unit)
- On a weekly basis, report all ESAVI, either Serious or Non-Serious in the surveillance bulletin / or other existing surveillance report.
- Compile all serious ESAVI cases and pass it on to the investigation team.
Figure 1: Flowchart of line of reporting
Figure 2: Serious versus Non-Serious ESAVI Decision Tree
Step 3. Investigation (Pharmacovigilance / Investigation team)
Once the ESAVI reports are received the following steps should be taken.
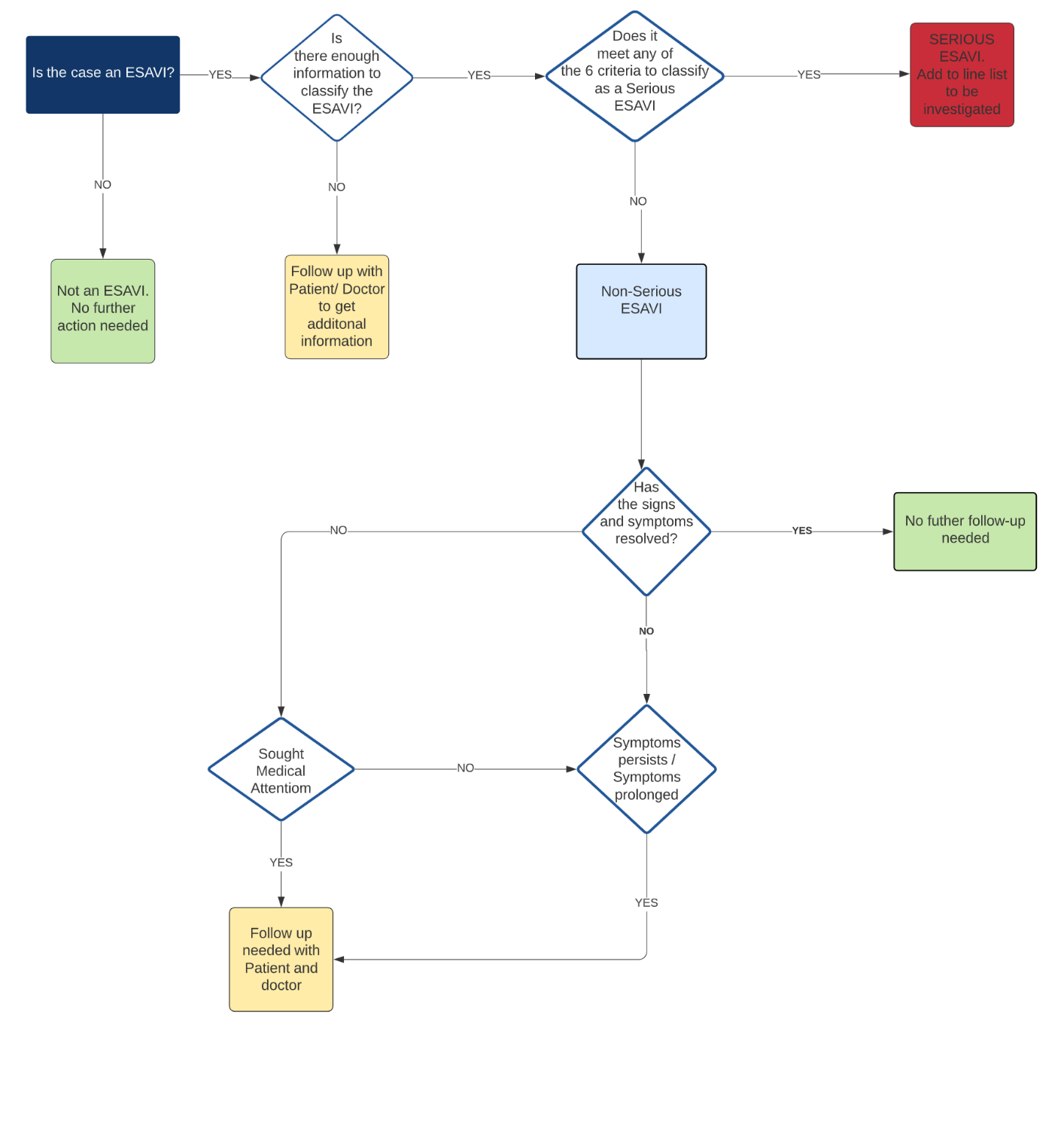
Reviewing cases
- Review the reported cases to identify any cases that are missing information.
- Contact the patient to verify and acquire any information that is missing.
- For any reports that were made by persons which indicates that medical attention was sought, or that the symptoms have not resolved, contact the patient, as well as the doctor for additional information.
Conducting an Investigation
- Confirm the investigation team comprising of – The Lead Investigator – medical physician, Public Health Nurse and Nursing Assistant.
- All required information should also be compiled from the person who notified the case(s).
- Contact the patient / next of kin to schedule an appointment to conduct the investigation within 24 hours after receiving notification.
- Preferably visit the patient / next of kin in the comfort of their home, or hospital if admitted, to conduct the investigation.
- If patient is deceased, get access of pathology / port-mortem / verbal autopsy report. If not done, ask for it to be done.
The following are the general steps for investigating an ESAVI (more detail is given in the regional ESAVI manual referenced below including specific instructions on investigating a cluster):
- Define the problem.
- Develop the plan for data collection and a structured organization for conducting the investigation.
- Consolidate clinical data, laboratory results, and information relating to the vaccine, the device used to administer it, and the diluent, where appropriate, including the storage conditions for these products.
All clinical data related to the event should be recorded:
- The chronological order of the appearance of signs and symptoms established
- The history of vaccination and of other diseases or interventions determined
- Any reactions or events associated with other immunizations identified
- Family history recorded
- Treatment and changes in the clinical picture described
- Pharmacological history
- Surgical history
- Toxicological history
- Gynecological history
- Housing and socioeconomic conditions
- A description of final health status
- In the event of death, an autopsy report
The vaccine data to be recorded include:
- The type of vaccine
- Manufacturer's name
- Exact lot number
- Expiration date
- Conditions of the diluent, if any
- Storage and transportation conditions (cold chain).
- Vaccine administration device and its characteristics
- Macroscopic feature of the vaccine, thinner, and administration device
- Macroscopic appearance of the vaccine after reconstitution
Vaccination process data to be recorded include:
- The date and time of administration
- The relation to the onset of symptoms
- The reconstitution procedure
- The origin and storage of vaccine inputs,
- Conditions of inputs for vaccination
- Vaccination staff
- Vaccination technique verified.
- Vaccination room and preparation area for administering the vaccine
- Program refrigeration equipment
- List of medicines received and delivered to the health service.
- Establish a timeline and identify facts related to the ESAVI.
- Identify factors related to the provision of the vaccination service.
- Identify group-related factors and those contributing to the ESAVI.
- Prepare the ESAVI investigation report and complete the corresponding investigation form.
- Submit the investigation report to the Vaccine Safety Committee/ Pharmacovigilance Team to determine causality.
- Plan a communication strategy based on the findings, as well as post-research activities, such as withdrawing a particular lot from the market and suspending a vaccination campaign.
Figure 3: Decision Tree to follow up with patients
Step 4. Data Analysis (Epidemiology Unit)
Clean all data and evaluate for completeness and accuracy- this includes correcting for misspellings. Complete the second phase of data cleaning and preparation in a statistical analysis software to ensure that all variables can be analyzed appropriately.
- Describe general summary measures (means, medians, standard deviations, percentages, etc.) of the ESAVI cases. These included distribution of the ESAVI cases by:
- Age
- Sex
- Parish
- Place of vaccination
- Vaccine Type
- Serious vs Non-serious
- Calculate incidence rates by:
- Type of adverse event
- Type of vaccine
- Age
- Sex
- Parish
- Number of doses administered
- Number of doses distributed
- Number of doses calculated based on coverage
- Number of individuals targeted
- Calculate the time period between the date of vaccination and the onset of symptoms as well as the time period between the onset of symptoms and the date of the report.
- To interpret observed ESAVI rates and behaviors over time, obtain two additional measures: Baseline incidence rate or background rate; Expected or known ESAVI rate.
Step 5. Causality Assessment (Vaccine Safety Committee / Pharmacovigilance Team)
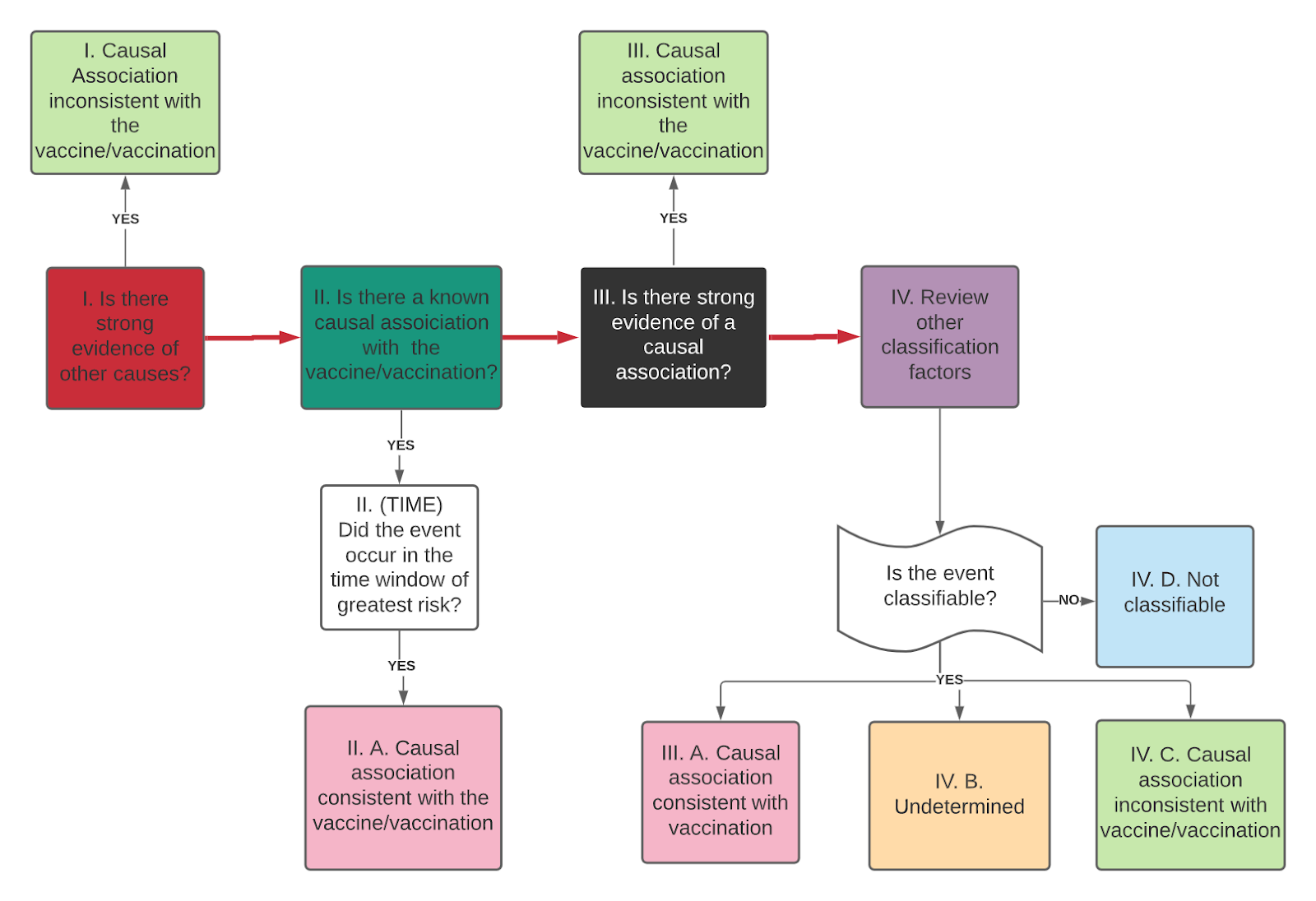
- Evaluation of eligibility - The case diagnosis must be confirmed and must meet standardized classification criteria, according to the Brighton Collaboration criteria (https://brightoncollaboration.us/category/pubs-tools/case-definitions/). In addition, the vaccine involved and the temporal relationship with the case should be verified in order to ensure that the vaccine was administered before the onset of symptoms or signs of the event. In order to perform the causality assessment, the investigation must first be completed and all details of the case made available. In this step, it is suggested that the question to be evaluated be defined and formulated.
- Verification checklist - A checklist should be used to evaluate the analytical elements and to ensure that all of the data collected during the investigation can be used.
- Is there evidence of other causes?
- Is there a known association with the vaccine or vaccination that is described in the medical literature?
- Is there evidence against a causal association?
- Have other factors (e.g. baseline event rate, health history, potential risk factors, medications, and biological plausibility) been considered?
The checklist includes four possible answers: Yes, No, Not known, Not applicable. If yes to any of the questions, comments and supporting evidence should be included.
- Causality assessment algorithm - Once the analytic questions have been answered, the algorithm is applied, making it possible to detect any trends in the evidence for the case. If it is not classifiable after applying the algorithm, it is recommended that all possible measures be taken to collect all of the missing information needed to classify it (Figure 3).
- Classification of the event (Possible classifications are as follows):
- Consistent causal association with the vaccine or with the vaccination process Undetermined
- No consistent causal association with vaccination (coinciding event).
- Non-classifiable
Figure 4: Causality Assessment Algorithm
Step 6: Feedback and Corrective Measures
The actions to be taken are according to the type of adverse event (see regional manual). Share / present findings backed by evidence immediately and in an appropriate manner with the patient involved once the course of action is identified and approved within the Vaccine Safety Committee / Pharmacovigilance Team.
References
1. Pan American Health Organization. Manual for Surveillance of Events Supposedly Attributable to Vaccination or Immunization in the Region of the Americas. Washington, DC: OPS. 2022