9.14. Rabies Lyssaviruses
Standard Operating Procedure for the Surveillance of Rabies Lyssaviruses
Effective Date: [Date to be Determined]
Version: 1.0
Prepared by: Dr. Maurice Frank, International PAHO Consultant, PAHO Barbados and ECC
Approved by: [Name/Department]
1. Introduction
- Purpose:
The purpose of this standard operating procedure (SOP) is to outline the procedures for the surveillance of rabies in a comprehensive step by step manner to ensure timely detection, reporting, and response to outbreaks in human populations. It defines the roles and responsibilities of all involved actors to ensure coordinated, effective multisectoral efforts. - Scope:
This SOP applies to all animal health, public health entities, healthcare providers, laboratories, and other stakeholders involved in viral zoonotic disease/rabies surveillance. - Definitions:
- Rabies: An almost always fatal, acute, progressive encephalitis caused by a lyssavirus transmitted to humans through contact with infected animals, via entry of virus-laden animal saliva via skin or rarely the mucous membranes.
- Surveillance: Systematic collection, analysis, and interpretation of health data.
- Legal Framework:
Reference to national, regional and international health regulations guiding the prevention, surveillance, treatment and control of rabies.
2. Key Actors Involved and Their Roles
2.1 Epidemiology Unit
- Role:
This is the central body responsible for overseeing the surveillance process in human populations. - Responsibilities:
- Develop national guidelines and protocols for rabies surveillance in human populations.
- Coordinate with the animal health unit, district health authorities, and laboratories on surveillance, detection, treatment and care.
- Provide training and resources to healthcare providers and other stakeholders.
- Analyse surveillance data and disseminate findings to various target audiences.
- Immediate reporting of syndromes of Fever and Neurological and suspected, probable and confirmed cases to regional and international bodies like PAHO, PAHO PANAFTOSA, and CARPHA.
- The timely completion and submission of case/outbreak reporting templates.
- To monitor the sustainable procurement and purchase of rabies vaccines for human populations and post-exposure prophylaxis, in collaboration with key partners (example- Expanded Programme on Immunization, Accident and Emergency Departments, etcetera).
2.2 Veterinary Public Health/ Animal Health Unit (Ministry of Health/ Ministry of Agriculture)
- Role:
The Veterinary Public Health/Animal Health Unit reports directly to the Epidemiology Unit, PAHO PANAFTOSA, Caribbean Animal Health and Food Safety Agency (CAHFSA), and the Caribbean Animal Health Network – Rabies Working Group (CaribVET) and is responsible for the complete reporting, and investigation of domesticated and wild animals demonstrating typical/atypical clinical signs of rabies/neurological symptoms. - Responsibilities:
- Utilise the national, regional and international rabies guidelines for domestic and wild animals.
- Implement, maintain, and enhance when required, routine active and passive surveillance systems for rabies lyssaviruses in domesticated and wild animal populations.
- Collaborate with national/regional wildlife ecologists and/or biologists to conduct sentinel surveillance of lyssaviruses and to monitor for potential spillover into terrestrial carnivores.
- Institute national prevention and control programs for rabies in domesticated and wild animals at designated ports of entry (PoE).
- Test for rabies lyssaviruses at designated accredited veterinary diagnostic laboratories.
- Conduct sample collection, storage, and submission in field-settings in accordance with internationally established guidelines for the submission of samples for rabies lyssavirus detection, to accredited veterinary diagnostic laboratories.
- Submit relevant surveillance data to the Epidemiology Unit.
- Coordinate with the designated laboratories for results and conduct timely and effective dissemination to relevant personnel and the national population.
- Inform animal owners of test results and follow-up with the Epidemiology Unit and/or relevant Health Units on appropriate post-exposure prophylaxis (PEP).
2.3 Health Units (HU) and Healthcare Providers (HCPs)
- Role:
The HU is the frontline body responsible for detecting and reporting suspected/probable rabies cases within the human population. - Responsibilities:
- Conduct reporting of Fever with Neurological symptoms to Epidemiology Unit within 24 hours.
- Report suspicion of suspect/probable rabies cases to the Epidemiology Unit, for patients presenting with clinical symptoms of encephalitis and animal exposure/ travel history to a rabies endemic country.
- Complete and submit the case investigation form to the Epidemiology Unit.
- Aid the Epidemiology Unit on community education on rabies.
- Conduct antemortem specimen collection.
- Conduct post-mortem specimen collection.
- Liaise with the laboratory for results.
- Administer post-exposure prophylaxis (PEP) to individuals exposed to rabid animals.
2.4 Expanded Program on Immunization
- Role:
- The Expanded Program on Immunization are responsible for monitoring the sustainable procurement and distribution of rabies immune globulin (RIG) and vaccines to public sentinel treatment and care sites.
- Responsibilities:
- Identify and address stockouts of rabies immune globulin (RIG) and vaccines.
- Guide healthcare providers on appropriate storage and administration of vaccines.
- Coordinate rabies vaccination efforts with high-risk individuals (veterinarians, laboratory technologists, abattoir staff).
2.5 Laboratories
- Role:
Laboratories play a crucial role in the confirmation of rabies cases. - Responsibilities:
- Provide instructions for sample collection and packaging in accordance with applicable guidelines.
- Receive samples and laboratory form; inform the relevant unit about rejection of sample as necessary.
- Conduct diagnostic testing for rabies.
- Report laboratory results to healthcare providers/ Epidemiology Unit (external laboratories) according to established reporting channels.
- Maintain quality controls in testing procedures.
- Share data with the Epidemiology Unit/ Veterinary Public Health Department/ Animal Health Unit and collaborate on epidemiological studies.
- Coordinate training on specimen collection, storage, and transportation.
2.6 Pathology Unit (Human and Animal)
- The Pathology Departments are responsible for conducting post-mortem in humans/ necropsies in animals to examine for gross lesions, and conduct specimen collection for histopathology and diagnostics.
2.7 Surveillance at Point of Entry
- The Veterinary Officer/ Immigration Officer is responsible for reviewing animal health records, including immunization history and results from accredited laboratories, prior to allowing animals entry into the country.
3. Surveillance Procedures
Step 1: Case Detection – Healthcare Providers / Laboratory Personnel
1. Immediate Reporting:
Report all suspected/probable cases to the Epidemiology Unit immediately for follow up. (Case definitions below)
Suspected rabies case definition
A suspected clinical case of rabies in humans is defined as: an acute neurological syndrome (i.e. encephalitis) dominated by forms of hyperactivity (furious rabies) or paralytic syndromes (paralytic rabies) progressing towards coma and death, usually by cardiac or respiratory failure, typically within 7–10 days of the first signs if no intensive care is instituted.
These may include any of the following clinical symptoms:
- Aerophagia
- Hydrophobia
- Paresthesia or localized pain
- Dysphagia
- Localized weakness
- Nausea
- Vomiting
- Some patients can present with atypical rabies, which includes a paralytic Guillain-Barre-like syndrome or other atypical features. (Commonly noted in cases exposed to bats or other wildlife.)
Probable rabies case definition
A suspected case plus a reliable history of contact with a suspected, probably or confirmed rabid animal.
Confirmed rabies case definition
A suspected or probable case that is confirmed in the laboratory.
2. Testing
Standard Diagnostic Tests for Rabies Lyssaviruses.
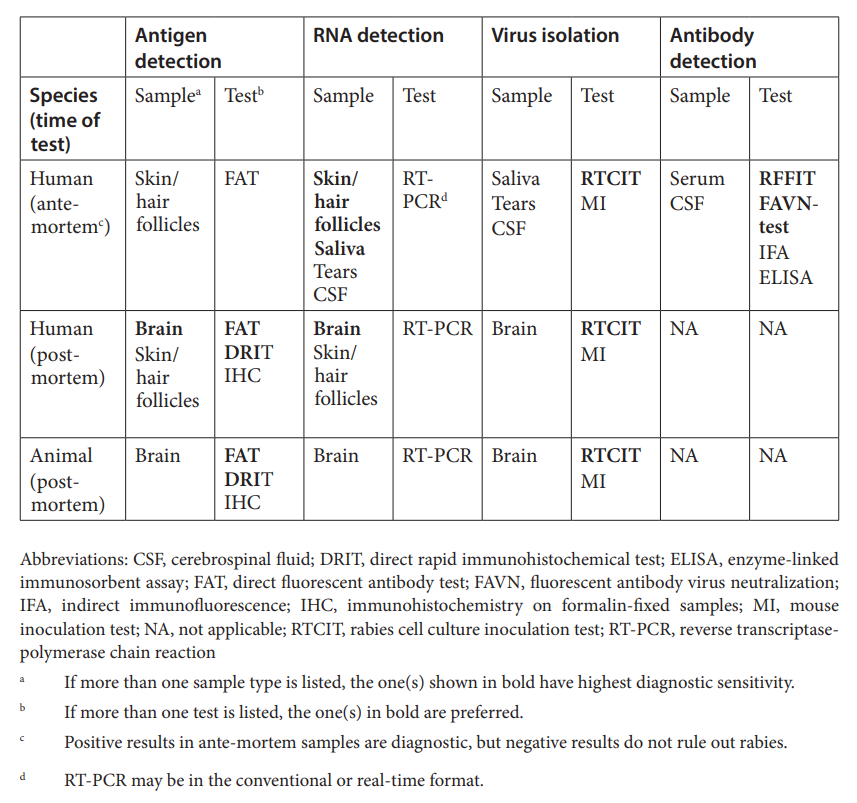
Source :
Ante-mortem diagnosis in humans-
- Secretions, biological fluids (such as saliva, CSF, tears, serum) and some tissues (such as skin biopsy samples, including hair follicles at the nape) can be used to diagnose rabies during life.
- The samples that afford the highest diagnostic sensitivity are at least three saliva samples, taken at intervals of 3–6 h, and skin biopsies (including hair follicles). Ideally, samples should be stored at –20 °C or less
Post-mortem diagnosis in humans/ Necropsy diagnosis in animals-
- Brain tissue is the preferred specimen for post-mortem diagnosis in both humans and other animals.
- Ideally, brain tissue should be kept refrigerated or frozen until testing. If this is not possible, samples can be preserved at ambient temperature in a 50% glycerine–saline solution.
- Freezing samples in glycerine is not recommended.
- The glyercine must be removed by washing prior to testing, and acetone fixation is not recommended before the direct fluorescent antibody test.
Transport of samples-
- Diagnostic specimens should be frozen or refrigerated, depending on the sample type.
- A cold chain should be maintained after sampling.
- Specimens for diagnosis of rabies should be shipped according to international regulations to avoid exposure.
- Packing instructions are given in the WHO recommendations on transport of infectious substances.
The recommended specimen types for laboratory testing of mpox virus:
- swabs of lesion surface
- exudate
- lesion crusts
Unroofing or aspiration of lesions (or otherwise using sharp instruments for mpox testing) before swabbing is unnecessary, and not recommended due to the risk for sharps injury. Lesions found in different phases (lesion surface or crust from healing lesion) can be swabbed.
3. Treat clients according to international guidelines
Note- Rabies is considered an overwhelmingly fatal disease. There is no effective curative treatment for rabies once clinical signs have appeared. Almost all patients with rabies will die.
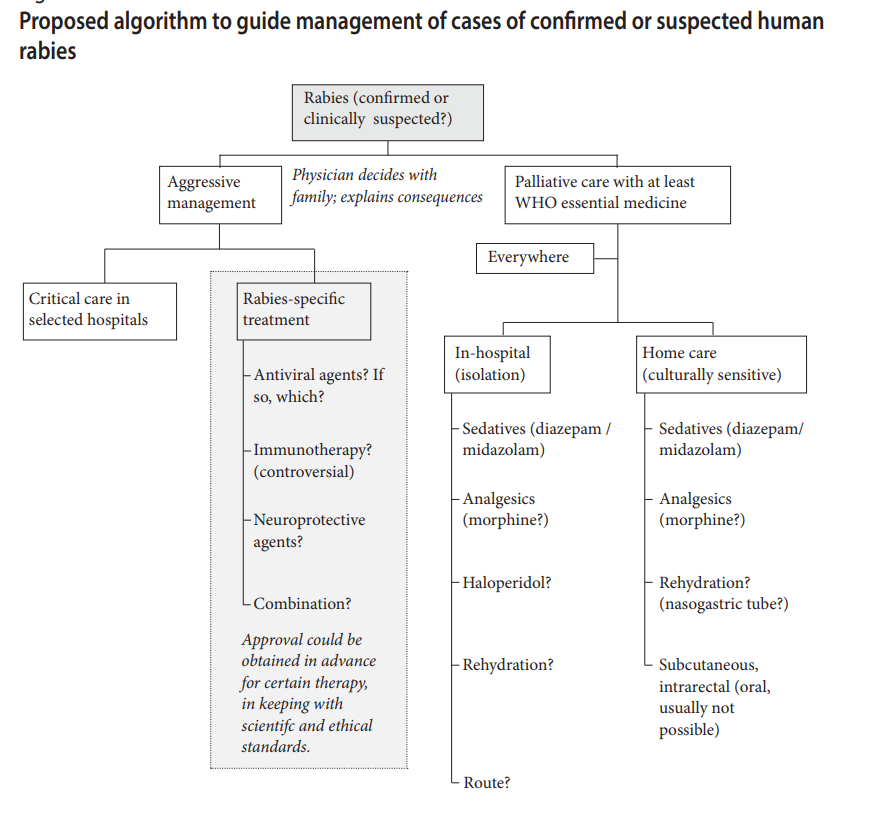
3.2 Recommendations for healthcare providers and family members of patients with rabies-
- Human rabies does not pose a risk to health care staff if routine precautions are taken, especially during intubation and suctioning.
- PEP should be provided for health care personnel considered to be at risk, after careful assessment, and they should be reminded of the importance of adhering to barrier nursing and wearing personal protective equipment (standard precautions, including wearing gloves, glasses and masks in case a procedure generates splashes).
- PEP may sometimes be necessary for the partners of patients, as close contact and sexual intercourse in the early stages of the disease pose a hypothetical risk for transmission (infectious rabies virus is present in saliva); however, no reports have clearly established human-to-human transmission.
- People exposed to the same biting animal should be identified and should receive adequate PEP.
- Breastfeeding to date has not been demonstrated as a route of transmission to infants.
- Patients are no longer eligible for organ transplant after death.
- The body of a suspected/confirmed case should be labelled as infectious but not as “contagious”.
4. Investigation for a suspect/probable/confirmed rabies case.
An investigation team may be made up of :
- 1 or 2 trained staff from Epidemiology Unit (strong consideration for persons trained in applied epidemiology).
- 1 veterinary epidemiologist/ surveillance officer for animal health/ chief veterinary officer (CVO) (strong consideration for persons trained in applied epidemiology).
- Health Education – Risk communication and community engagement (RCCE)
- Pathologist (Human and Animal Pathologists) to conduct relevant post-mortem/necropsy examinations and specimen collection.
Deliverables of the investigation team :
Investigation of suspected cases
- Completion of the investigation form
- Identification of exposure to the rabid animal
- Collection, conservation and transport of specimens
- Submit the investigation forms to relevant regional entities (outlined previously).
Contact tracing
- Locate and follow up on contacts (home visit, telephone follow-up)
- Review travel history for travel to endemic rabies countries.
- Preliminary assessment of each contact with the rabid animal to review need for PEP.
- Navigation of persons exposed to rabid animals to Health Unit for PEP.
- Submission of animal samples to the laboratory for necropsy and diagnostics.
- Submit information collected to relevant entities
4.1 Data Management and Dissemination
- Data should be cleaned and analysed between both human and animal health departments.
- Data should be disseminated on a shared, secured platform.
- Preparation of a case report should be done on the evolution of the epidemiological situation of the disease.
- Dissemination of an immediate press release to the local and regional media should be conducted, upon confirmation of a positive rabies diagnosis.
- Complete reports must be completed and submitted entailing both human and animal health information/ travel history, to the relevant human and animal health units at the regional and international levels.
